Second human trials of Covid-19 vaccine expected to start this month
If all three phases show good results, the second made-in-Vietnam Covid-19 vaccine will be available in the
The Covivac vaccine is the research work of the Institute of Vaccines and Medical Biologicals (IVAC) under the Ministry of Health (MOH). It had earlier yielded safe results and a strong immunity responses on mice, rabbits, etc. Given promising results on animals, the vaccine is granted to enter human trials starting January, two months earlier than expected.
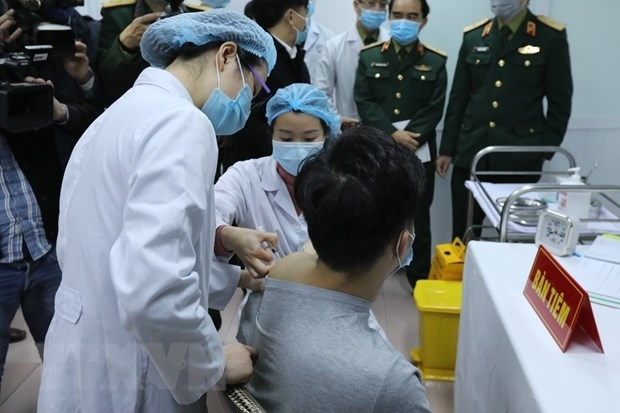
As reported by VOV, IVAC will cooperate with the National Institute of Hygiene and Epidemiology and Hanoi Medical University to trial the vaccine on 125 volunteers aged 18-59. Those receiving IVAC’s trial jabs must be healthy, having no underlying disease and satisfying several other specific criteria.
The first batch of volunteers will be divided into different groups who receive different doses. Following stages will be conducted if the human trials go smoothly.
According to Dr. Duong Huu Thai, Director of IVAC, the Institue has been studying Covivac since last May, aiming at successfully producing the vaccine and completing three human trials within 18 months. The first phase of the trails is scheduled to finish in April.
“If all three trial phases yield good results, Vietnam might have the vaccine in late 2021”, Thai said. “IVAC has an edge in the production process thanks to its available infrastructure, technology, and decade-long experience in producing flu vaccines”.
Earlier on December 10, Nanogen’s COVID-19 vaccine was the first of its kind indigenous in Vietnam to enter human trials. Nanogen Biopharmaceutical company is expected to end its Nanocovax vaccine’s human trials by February 2022.
The human clinical trials protocol, which includes three phrases, was approved by the Ethics Council of the Ministry of Health on December 9.
Each phrase consists of two injections, 28 days apart.
The first phase aiming at testing the safety of Nanocovax started on December 10 and is scheduled to end in February 2021. It was conducted in Hanoi on 60 volunteers aged 18-50. The first three volunteers who received the jab on December 17 showed no abnormal symptoms to date.
The second phase is planned to start right after the first one and last for 6 months. 400-600 volunteers aged 12-75 will be recruited for the trial, which evaluates the immunogenicity and preventive potency of the vaccine.
The third phase (August 2021 – February 2022) is planned to take place in an epidemiological area in India, Indonesia, or Bangladesh to gather enough 1,500 – 3,000 volunteers aged 12-75.
Researchers said they might kick off the second phase earlier right after the first phase yields 50 percent effective. Thus, according to Do Minh Si, Development Research Director of Nanogen, the clinical trials might end at the end of 2021.
Nanocovax is priced at VND120,000 ($5.17) per dose.
Along with injections, Vietnam’s COVID-19 Nanocovax vaccine will also be developed in the form of eye-drop and nasal spray for special subjects.
Vietnam has four Covid-19 vaccines produced by Nanogen, Vabiotech, Polyvac and the Institute of Vaccines and Medical Biologicals (IVAC) currently under research.
Vabiotech, Polyvac are currently evaluating their vaccines on animals, having completed the laboratory-scale production process.
On global scale, there are currently 11 COVID-19 vaccine candidates under the third phase of human trials. Pfizer/BioNTech’s vaccine (the US) is the first vaccine to complete the trials with 95 percent effectiveness and granted the emergency use authorization from the UK and Bahrain.
Meanwhile, Moderna’s vaccine is on its final clinical trial phase, with effective rate reaches 94.5 percent. Oxford/ AstraZeneca is 70-90 percent effective, depending on the injection dose. Russia’s Sputnik V (95 percent effective) is scheduled to begin mass vaccination next week.
Moderna’s vaccine is priced at 37 USD per dose, meanwhile, Pfizer’s vaccine and Oxford’s vaccine are more reasonably priced at 19 USD and 3 UDD per dose, respectively.